No one likes being ill but it’s just a part of life that we must endure, hopefully not regularly. However, being ill can disrupt the composition and diversity of your gut microbiome, contributing to a phenomenon called dysbiosis, especially if you’re given antibiotics to rid the infection from your body.
Here, we’ll recap the gut microbiome and dysbiosis before exploring a study conducted by Button et al. (2023) investigating the effect of an HMO synbiotic on antibiotic-induced imbalanced adult gut microbiomes.
What is the gut microbiome?
The human gut microbiome is a complex ecosystem made up of trillions, yes trillions, of tiny microbes all housed within your digestive tract. These microbes have key roles in supporting and maintaining your health both within the gut and around your body.
Your gut microbiome is almost as old as you, with the foundations of its growth being laid during early infancy. If you were born naturally i.e., via your mother’s vagina, you would have been seeded with a greater number of beneficial bacteria, including:
- Bacteroides
- Bifidobacteria
- Lactobacillus[i]
These colonies can be further enhanced through a diet of breastmilk when Bifidobacteria become a prominent species[ii]. As you grow, your diet and other environmental factors can influence what your microbiome looks like and how it functions.
Overall, your gut microbiome is vital for digestive processes, supporting the immune system, protecting you against illness and inflammation, and even boosting your mood.
Dysbiosis and the need for balance
When it comes to a healthy gut microbiome, balance is key. The gut microbiota plays a significant role in the development of most human diseases if it is caught off-kilter. Most illnesses are associated with disruptions in the composition and function of the bacterial colonies residing in the gut, also known as dysbiosis[iii].
Common features of dysbiosis
Typically, dysbiosis is associated with at least one of the following:
- A reduction in microbiota diversity
- A loss of good bacteria
- An increased growth of harmful or pathogenic bacteria
What causes dysbiosis?
Dysbiosis has been linked to a wide variety of conditions, including:
- Crohn’s Disease
- Ulcerative colitis
- Autism
- Obesity
- Colorectal cancer
- Allergies
- Type 1 and 2 diabetes[iv]
But what really causes it?
An imbalanced gut can be caused by environmental factors, genes, or even your lifestyle. For example, a diet high in sugar and low in fibre can promote an environment where harmful bacteria dominate, triggering inflammation and a weakened intestinal barrier[v]. Taking antibiotics can also disrupt your microbiome by ridding your gut of its health-promoting residents.
All these factors can stir up a recipe for disaster that not only directly impacts your gut health, but can have a wider, detrimental effect on the rest of your body and wellbeing.
How can we restore balance?
To limit the trauma of dysbiosis on your body, there are things you can do to help restore calm. Many of the strategies proposed in the scientific literature include the administration of probiotics (health-promoting microbes), prebiotics (the indigestible fibre that acts as a food source for probiotics), and synbiotics (a combination of pre-and probiotics).
Here are some of the ways you can help support your gut and restore harmony:
- Probiotics: Several studies have shown that probiotic bacteria can modulate the composition of the gut microbiome. Bifidobacterium longum longum BB536 demonstrated in a 2019 study that it could improve immune dysfunction and drive homeostatic balance between the host and its microbiome[vi].
- Prebiotics: Prebiotics have numerous roles in protecting and maintaining human health. As a food source for probiotic bacteria, they are fermented into important metabolites like short-chain fatty acids which can benefit the gastrointestinal, immune and nervous systems. They also help to maintain the growth and activity of good bacteria, keeping pathogens at bay[vii].
- Synbiotics: A careful combination of prebiotics and probiotics has been demonstrated in numerous studies to have a positive effect on human health. A study by Chen et al. (2020) found that combining xylooligosaccharide and Lactobacillus rhamnosus reduced the Firmicutes/Bacteroidetes ratio (associated with the development of metabolic disease) and enhanced the recovery of beneficial bacteria in the gut[viii].
- Gut farmer diet: Diet is a key modulator of the gut microbiome. Too much sugar, fat, and not enough fibre can disrupt its composition, leaving you at risk of inflammation and illness. Dietary methods like the gut farmer diet can help maximise the output of your gut microbiome and it's simple to follow.
What are synbiotics?
As we mentioned, one of the ways balance can be restored is through the administration of synbiotics.
Synbiotics are:
“a mixture comprising live micro-organisms and substrate(s) selectively utilised by host micro-organisms that confers a health benefit on the host.”[ix]
Synbiotics are clever because they take advantage of the synergistic relationship between probiotics and prebiotics. Together, they work in harmony to promote the health of your gut, and both are naturally present in your diet, even if you did not know it.
Although they can bring about enhanced health benefits, not all synbiotics are created equal. Some probiotic strains are better researched than others, while some prebiotics are more suited to specific strains. It’s about finding what works best for you, but novel synbiotics are being tested, including those containing human milk oligosaccharides (HMOs).
Reshaping the gut microbiome after illness using an HMO synbiotic
The human gut microbiome is a complex ecosystem which is influenced by several environmental factors that can not only do good, but can also have negative consequences, including dysbiosis.
One way to prevent this from occurring or even reversing disease, is by manipulating the imbalanced microbiome using live biotherapeutic products (LBPs). The use of synbiotics, the pairing of health promoting bacteria with their preferred nutrient source (prebiotics), is one way this may be achieved.
Button et al. (2023) conducted a study to assess if a synbiotic comprising of human milk oligosaccharides and the gut microbe Bifidobacterium longum subspecies infantis (B. infantis) found in the breastfed infant gut, could reverse a state of dysbiosis.
How was the study conducted?
The study comprised a cohort of 56 healthy volunteers who were assigned to three different cohorts. All 56 volunteers were given antibiotics to induce a state of dysbiosis.
The three cohorts were as follows:
- 19 volunteers were given antibiotics only (control group)
- 17 volunteers were assigned antibiotics and infantis only
- 17 volunteers were given antibiotics and infantis and HMOs (synbiotic)
The trial was conducted over a 35-day period as shown in the chart below:
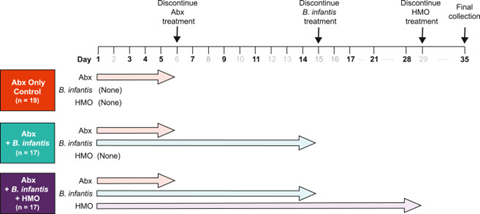
Source: Button et al. (2023)
What did results show?
The study by Button et al. (2023) investigated the engraftment of B. infantis in the gut microbiome of healthy subjects after being exposed to antibiotics, and if HMOs can promote engraftment of healthy bacteria.
The results showed that after antibiotic treatment to induce a temporary state of dysbiosis was stopped, the engraftment of B. infantis was high in 76% of subjects receiving the HMO synbiotic. Thus, highlighting the importance of combining prebiotics and probiotics for enhanced health results.
As well as engraftment of B. infantis, the results showed that volunteers who received the B. infantis and HMO synbiotic also had an enriched abundance of Veillonella.
Veillonella is a commensal bacteria found in both the gut and mouth microbiomes. Its increased abundance suggests that cross feeding occurred in volunteers who were assigned the B. infantis and HMO synbiotic.
Veillonella is known to feed on lactate and produce the short chain fatty acids (SCFAs) acetate and propionate which support glucose and immune homeostasis. The increased abundance of Veillonella in the synbiotic volunteers was also supported by results using mouse models.
The effect of B. infantis and HMOs and gut microbiome structure and activity
The study also examined the relationship between the HMO-dependent engraftment of B. infantis and microbially produced metabolites. It found that there was an increase in indole-3-lactic acid (ILA).
Studies have shown that ILA can have anti-inflammatory, immunomodulatory, and immune protective benefits and is found in Bifidobacteria dominant gut microbiomes[x]. Button et al. (2023) also demonstrated that the HMO-dependent engraftment of B. infantis resulted in a decrease in pro-inflammatory molecules, such as:
- pCS
- pCG
- Phenol sulfate
A reduction in these molecules is a positive benefit because when they are elevated, they are associated with intestinal barrier dysfunction and kidney disease.
Summary and future considerations
Overall, the study showed that HMO-dependent B. infantis engraftment during a temporary state of dysbiosis could induce changes in the composition of the microbiome and its activity which could positively benefit the host.
HMOs are believed to have evolved to promote a high abundance of Bifidobacteria in the infant gut, a keystone species known to help develop and support the human gut microbiome. A microbiome dominant in Bifidobacteria can have positive effects on the immune system and can protect the host from gastrointestinal pathogens.
These benefits may also be seen in adult gut microbiomes where dysbiosis may be occurring. Therefore, the study by Button et al. (2023) demonstrate that an HMO and B.infantis synbiotic, may promote recovery following antibiotic induced dysbiosis.
Are you looking to support your own gut health? Check out our range of PureHMO® products and prebiotics like Organic Apple Peel Powder and Simple Reds
Written by: Leanne Edermaniger, M.Sc. Leanne is a professional science writer who specializes in human health and enjoys writing about all things related to the gut microbiome.
Sources
[i] Coelho GDP, Ayres LFA, Barreto DS, Henriques BD, Prado MRMC, Passos CMD. Acquisition of microbiota according to the type of birth: an integrative review. Rev Lat Am Enfermagem. 2021 Jul 19;29:e3446. doi: 10.1590/1518.8345.4466.3446. PMID: 34287544; PMCID: PMC8294792.
[ii] Martín R, Jiménez E, Heilig H, Fernández L, Marín ML, Zoetendal EG, et al. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Applied and Environmental Microbiology. 2009;75(4):965–9. doi:10.1128/aem.02063-08
[iii] Hrncir T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms. 2022 Mar 7;10(3):578. doi: 10.3390/microorganisms10030578. PMID: 35336153; PMCID: PMC8954387.
[iv] DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm Bowel Dis. 2016 May;22(5):1137-50. doi: 10.1097/MIB.0000000000000750. PMID: 27070911; PMCID: PMC4838534.
[v] Martinez JE, Kahana DD, Ghuman S, Wilson HP, Wilson J, Kim SC, et al. Unhealthy lifestyle and gut dysbiosis: A better understanding of the effects of poor diet and nicotine on the intestinal microbiome. Frontiers in Endocrinology. 2021;12. doi:10.3389/fendo.2021.667066
[vi] Wong CB, Odamaki T, Xiao J. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. Journal of Functional Foods. 2019;54:506–19. doi:10.1016/j.jff.2019.02.002
[vii] Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A, Ghasemi Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods. 2019 Mar 9;8(3):92. doi: 10.3390/foods8030092. PMID: 30857316; PMCID: PMC6463098.
[viii] Li C, Niu Z, Zou M, Liu S, Wang M, Gu X, et al. Probiotics, prebiotics, and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms. Journal of Dairy Science. 2020;103(7):5816–29. doi:10.3168/jds.2019-18003
[ix] Hancocks N. International panel of scientists agree what makes a “synbiotic” [Internet]. William Reed Ltd; 2020 [cited 2023 Dec 18]. Available from: https://www.nutraingredients.com/Article/2020/08/24/International-panel-of-scientists-agree-what-makes-a-synbiotic
[x] Ehrlich, A.M., Pacheco, A.R., Henrick, B.M. et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol 20, 357 (2020). https://doi.org/10.1186/s12866-020-02023-y








