The microbiome encompasses all of the microbes and viruses that live in and on our bodies. Specifically, the colon contains the largest ecosystem, housing upwards of 100 trillion bacteria. Advances in research continue to reveal the significant link between these microbial communities and disease, with different microbiome signatures linked to a variety of illnesses.
Furthermore, we now understand that the microbiota also plays a vital role in the maintenance of health, creating metabolites that interact with host tissues and modulate immune cells, support anti-inflammation, and promote healthy metabolic function.
Cardiovascular disease (CVD) comprises a variety of illnesses with largely metabolic underpinnings. These include:
- Atherosclerosis
- Ischemic heart disease
- Stroke
- Heart failure
CVD is the leading major cause of death globally, and rates of CVD have steadily increased over the decades. Alarmingly, rates of subclinical atherosclerosis have also been climbing among middle-aged populations. This information tells us two things 1) the underlying cause(s) of atherosclerosis are affecting increasingly younger populations and 2) atherosclerosis begins decades before an individual has a cardiac event.
There are a variety of risk factors for CVD that can be broadly categorized into modifiable and non-modifiable. Modifiable risks include nutrition, exercise frequency and duration, and smoking, whereas non-modifiable risks include genetic predispositions. CVD is typically preceded by multiple physiological and metabolic aberrations:
- High levels of visceral fat (i.e. fat around the organs within the belly)
- Poor blood lipid profiles (i.e. high triglycerides and low HDL cholesterol)
- Insulin resistance
- High blood pressure
Together these aberrations are frequently referred to as metabolic syndrome. CVD and metabolic syndrome are both characterized by increased levels of inflammation throughout the body, so understanding the causes of this inflammation and finding ways to mitigate these effects is imperative.
In a recent Nature review article entitled “The potential of tailoring the gut microbiome to prevent and treat cardiometabolic disease”, Chakaroun et al outline the field’s current understanding of the link between the microbiome and CVD, and explore the ways in which this may be leveraged for health outcomes.
Research in animal models has revealed that CVD is transmissible via fecal matter transplant (FMT). As the name implies, FMT involves the transfer of stool from one animal into another. In these experiments, researchers took stool from animals with diet-induced metabolic syndrome and CVD and transferred it into healthy animals. The result: the healthy animals developed metabolic syndrome and CVD. This suggests that the microbiome plays a causal role in the development of these diseases. Importantly, however, the reverse has also been shown to be true—transferring stool from a healthy animal into a diseased animal resulted in amelioration of illness.
In response to compelling findings such as these, a large interest in gut microbe-based therapeutics has been garnered within field. One of the most striking clinical proof-of-concepts is the use of FMT in patients with recurring C. difficile infection. In this patient population, FMTs are largely curative. For the treatment of non-infectious illnesses, however, the effects of FMTs have been largely underwhelming, producing mild and transient results.
The Role of the Microbiome in Cardiometabolic Disease
Obesity
The astronomical increases in obesity rates over the past several decades points to the significant disparities between our bodies and the modern environment. Our physiology and biochemistry evolved in the ancestral environment, where features like processed foods, synthetic chemicals, and 9-to-5 life didn’t exist. Research exploring the causal links between the microbiome and obesity has revealed that obesity is frequently associated with lower diversity and species richness within the gut microbiome, but that these effects are not consistent across the breadth of the literature. However, studies comparing metabolically healthy and unhealthy obese individuals illustrate that loss of microbiome diversity is one of the early signs of the onset of metabolic syndrome.
A strong link has been observed between BMI and the presence of the microbe Christensenella minuta, where higher levels of C. minuta are associated with lower visceral fat and decreased plasma triglycerides and apolipoprotein B levels. Moreover, weight loss is associated with increased levels of C. minuta and, in rodent models, FMT from mice possessing C. minuta into overweight germ-free mice led to enhanced weight loss.
Additionally, individuals expressing high levels of microbial gene content appear to be protected against metabolic syndrome. In particular, enrichment of the following short chain fatty acid (SCFA)-producing bacteria are associated with protection
- Roseburia
- Akkermansia
- Actinobacteria like Bifidobacteria
Conversely, enrichment of Proteobacteria, pathogenic strains, and increased expression of the enzymes peroxidase and catalase are associated with lower bacterial gene content and increased risk of disease. In particular, these features are indicative of elevated oxygen levels within the colon: an environment that is anaerobic (i.e. oxygen-depleted) under healthy conditions.
Insulin Resistance and Type 2 Diabetes
Type 2 diabetes (T2D) is linked to broad changes in the composition of the gut microbiome including:
- Decreased species richness
- Decreased levels of butyrate-producers
- Increased levels of opportunistic pathogens
- Increased levels of Veillonella
Veillonella is a lactate-consuming bacteria associated with both visceral fat and insulin resistance in individuals across disparate cultures and environments. Importantly, the decline in butyrate producers like Roseburia, Faecalibacterium, and Clostridium is more significant than the increase in pathogen levels. This observation suggests that supporting butyrate-producing strains likely confers a protective effect against disease progression and helps to stabilize the ecosystem within the gut. In addition to reduced butyrate production, there are other metabolic signatures of the T2D microbiome
- Increased branched chain amino acid production
- Increased sugar breakdown
- Increased lactate and B vitamin production
However, the role of these changes in T2D pathogenesis is unclear.
Hyperlipidemia
An inverse relationship between gut microbiome diversity and blood triglyceride and LDL cholesterol levels in individuals with metabolic syndrome has been reported consistently in the literature. On the other hand, higher levels of microbial diversity are positively associated with HDL cholesterol levels. Specifically, Eggerthella enrichment was shown to correspond to increased triglyceride and decreased HDL cholesterol levels. Meanwhile, enrichment of Bacteroidales and Clostridiaceae showed the opposite, as these bacteria directly contribute to bile acid and SCFA metabolism.
Furthermore, it is well-established that the microbiome plays an important role in cholesterol metabolism, where some microbes have the ability to convert cholesterol into non-absorbable metabolites that are then excreted via the stool. However, it is unclear as to whether these contributions have a meaningful impact on serum cholesterol metrics.
Hypertension
Hypertension is the most common and treatable risk factor for CVD. In laboratory models, direct links have been shown between the gut microbiome, levels of bacterial metabolites, and blood pressure.
Furthermore, hypertension-linked microbe signatures correspond to increased arterial stiffness. Multiple studies have shown that hypertension is associated with depletion of the butyrate producers
- Roseburia
- Faecalibacterium
- Several species of Ruminococcaceae
- Christensenella
Unpacking the Data
Large studies have been conducted that have sought to adjust metabolomic and bacterial sequencing data for polypharmacy in an attempt to understand the effects of commonly prescribed drugs on data interpretation. Corroborating previous findings, these studies showed that the decline in butyrate-producing species of bacteria precluded any overt signs of CVD, arising during the early insulin resistance phase of pathogenesis. These results suggest that it may be prudent for at-risk individuals to focus on optimizing for these commensal bacteria to slow down or reverse disease progression.
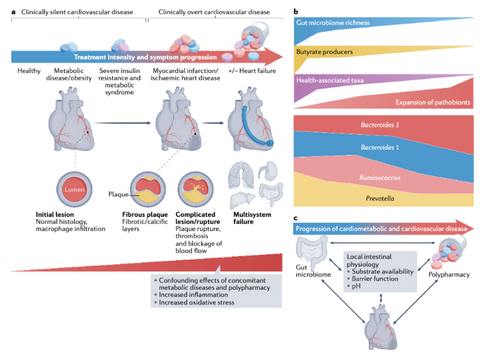
Figure 1. CVD Disease Progression [1]
Nutritional Considerations
There are four major nutritional factors that are highlighted in the CVD literature:
- Prebiotics
- Trimethylamine oxide (TMAO)
- Imidazole propionate
- Bile acids
Prebiotics
The consumption of prebiotics (i.e. indigestible carbohydrates) like dietary fibers, resistant starches, and polyphenols promote the production of SCFAs in the colon by key microbial species. The consumption of foods containing these molecules preferentially promotes the growth of these species and, thus, may play an important role in a CVD prevention strategy.
TMAO
A metabolite of carnitine and choline know as trimethylamine oxide (TMAO) has gotten a great deal of press over the past decade due to its correlation to increased CVD risk. The microbiome facilitates the conversion of carnitine and choline from animal foods like eggs and fish into TMAO, and the microbiomes of vegetarians and vegans have been shown to possess less capacity to conduct this biochemical conversion. Animal studies appeared to corroborate these epidemiologic findings. However, large cohort studies in humans over the past several years have failed to demonstrate a direct causal link between TMAO and CVD. In fact, Nordic and Mediterranean populations have among the highest TMAO blood levels but experience far less CVD than individuals in the West. Moreover, some studies have shown neutral or even beneficial effects of TMAO in CVD models. Thus, there is not currently a clear understanding as to the role that this metabolite plays in CVD pathogenesis, and the avoidance of foods rich in carnitine and choline to mitigate TMAO production is not warranted.
Imidazole Propionate
Another molecule of interest within the topic of CVD is imidazole propionate—a metabolite formed during the breakdown of the essential amino acid histidine. This metabolic conversion is unique to bacteria, and individuals with insulin resistance and T2D possess microbiomes with an increased ability to generate imidazole propionate. Furthermore, blood levels of imidazole propionate are closely linked to clinical markers for glycemic control and inflammation (i.e. HbA1C and C-reactive protein, respectively). Increased levels of the precursor to imidazole propionate have been identified in response to decreased species richness and diversity in individuals with pre-diabetes. As a result, researchers believe that this molecule may play a causative role in metabolic dysfunction and hope to target its production pharmacologically. Additionally, levels of this metabolite correspond to a Bacteroides 2 enterotype (characterized by high Bacteroides relative to Faecalibacterium), and low butyrate production. Thus, dietary approaches geared towards the cultivation of SCFA-producing bacteria will likely lead to a reduction in imidazole propionate levels.
Bile Acids
Bile acids help to emulsify dietary fat and facilitate its absorption from fat-containing meals. Elevated circulating bile acids are observed in liver disease and CVD and are largely predictive of coronary artery disease in human studies. Bile acids can undergo metabolic conversion by microbes within the gut into molecules referred to as secondary bile acids. Some of these secondary metabolites are subsequently excreted in the stool, while others can enter the host circulation and interact with cellular receptors, some with positive and some with negative effects. Having a diet and lifestyle that promotes liver health and prevents or reverses liver fat accumulation is important to maintain healthy bile acid levels and a well-functioning metabolism. Avoiding processed foods, particularly those containing added sugars and high fructose corn syrup, will help to protect the liver from excess fat accumulation.
References
[1] Chakaroun RM, Olsson LM, Bäckhed F. The potential of tailoring the gut microbiome to prevent and treat cardiometabolic disease. Nat Rev Cardiol. 2022 Oct 14. doi: 10.1038/s41569-022-00771-0. Epub ahead of print. PMID: 36241728.








